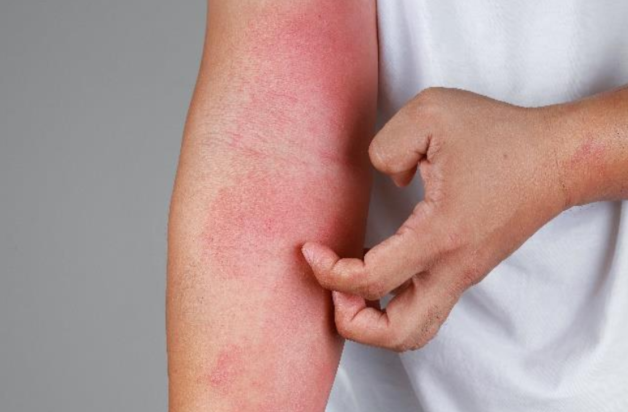
Do you have Atopic Dermatitis?
Atopic dermatitis (AD) is the most common form of eczema. It is a chronic condition that causes dry, itchy, and inflamed skin. This skin disorder affects individuals of all ages, ethnicities, and all geographic regions worldwide.
We are currently recruiting people aged 18 to 75 years with moderate to severe atopic dermatitis, to participate in an investigational study called the COMPASS 2-AD study.
The COMPASS 2-AD study consists of an injection under the skin every two weeks for 30 weeks. This treatment is experimental. There will be three groups in this study, and if you are eligible to participate, you will be randomly assigned to one of these groups (like drawing numbers from a hat). Everyone who participates in the study will receive both the study drug and the placebo, but the doses and the order in which the drug or placebo is received will vary depending on the group you are assigned to.
Compensation for your time and travel may be available. There will be no cost to you for being considered or for taking part in this study, and you will be provided with all study medication, examinations, and medical care related to the study at no cost to you.
Join the trial to contribute to the development of this treatment and potentially benefit from its effects.





