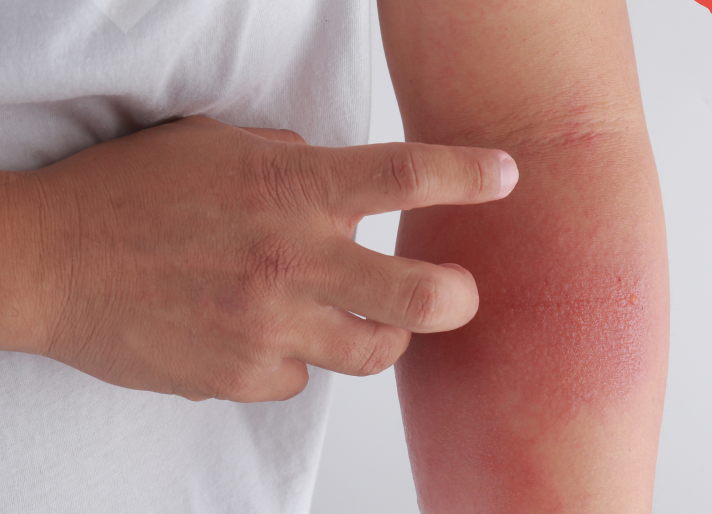
Atopic dermatitis (AD), the most common form of eczema, is a chronic inflammatory skin disorder that affects individuals of all ages, races, and geographic regions worldwide.
We are currently recruiting participants 18 years and older with moderate-to-severe eczema, also known as atopic dermatitis, to participate in a clinical study. This study is being done to evaluate the safety and efficacy (how well the drug works) of an injectable (like a shot) drug known as APG777.
Study participation will last approximately 2 years and comprises of 22 study visits. The study will include of 52 weeks of treatment followed by approximately 48-52 weeks of follow-up period. For the first 16 weeks of treatment, participants will have a 2 in 3 (66.6%) chance to receive APG777 and 1 in 3 (33.3%) chance to receive the placebo (shots with no study drug). After 16 weeks of treatment, all participants will receive APG777.
Visits take approximately 2 to 4 hours depending on which tests need to be completed during the visit.
Blood samples will be collected from all participants at the first visit and throughout the study.
An electrocardiogram to check heart health will be taken at visit 1 and medical photography will be used to evaluate your eczema over time. An eye exam will also be performed at visit 1 and at the end of the study as part of a comprehensive safety evaluation during this study.
Compensation for time and travel may be available. There will be no cost to you for taking part in this study and you will be provided with all study medication, examinations and medical care related to the study.
See if you are eligible for the study!





